The blog presents the short Questions and Answers of the Chapter 3, Chemical Reactions and Equations, from Science Part I of Class 10 from Maharashtra State Board. These can be very useful for students preparing for SSC Board exams as well as competitive exams, as it includes the objective type basic concepts & fundamentals.
Q17. What are the factors affecting the rate of reaction ?
Ans. Factors affecting the rate of a chemical reaction are :
(a) Nature of the Reactants
(b) Size of the Particles of Reactants
(c) Concentration of the reactants
(d) Temperature of the Reaction
(e) Catalyst
Q18. What is a catalyst ?
Ans. The substance in whose presence the rate of a chemical reaction changes, without causing any chemical change to it, is called a catalyst.
Q19. What is Oxidation Reaction ?
Ans. The chemical reaction in which a reactant combines with oxygen or
loses hydrogen to form the product is called oxidation reaction.
Examples :
Q20. What are oxidants or oxidizing agents ?
Ans. Chemical substances which bring about an oxidation reaction by making oxygen available are called oxidants or oxidizing agents.
Q21. Give exampes of oxidizing agents ?
Ans. The examples of oxidizing agents are :
Q22. What is Reduction Reaction ?
Ans. The chemical reactions in which reactants either gain hydrogen or loses oxygen to form the product are called reduction reactions.
Q23. What are reductant or reducing agents ?
Ans. The substance that brings about a reduction reaction is called a reductant, or a reducing agent.
Q24. What is Redox Reaction ?
Ans. Reactions in which the oxidation and reduction reactions occur simultaneously are called as Redox Reactions. The reductant is oxidized by the oxidant and the oxidant is reduced by the reductant.
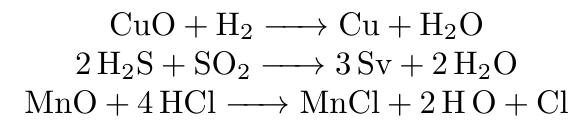
Q25. What do you mean by Oxidation ?
Ans. When the positive charge on an atom or an ion increases or the negative charge on them decreases it is called oxidation.
Q26. What do you mean by Reduction ?
Ans. When the positive charge decreases or the negative charge increases it is called reduction.
Q27. Give an example of redox reaction ?
Ans. A redox reaction takes place during cellular respiration.
Q28. What is rust, write its chemical formula ?
Ans. A rust is a certain type of reddish coloured solid layer that collects on their metallic surface. Its chemical formula is Fe2O3 X H2 O.
Q29. How is rust formed ?
Ans. The rust is formed by an electrochemical
reaction. Different regions on the surface of iron become anode and cathode.
Q30. What is corrosion ?
Ans. Due to various components of atmosphere, oxidation of metals takes place, consequently resulting in their damage. This is called corrosion.
Q31. What is rancidity ?
Ans. When we use old, left over cooking oil for making food stuff, it is found to have foul odour called rancidity.
Q32. How rancidity can be prevented ?
Ans. (i) Rancidity in the food stuff cooked in oil or ghee is prevented by using antioxidants.
(ii) The process of oxidation reaction of food stuff can also be slowed down by storing it in air tight container.
************************
Discover more from Dr. Ganesh Visavale
Subscribe to get the latest posts sent to your email.

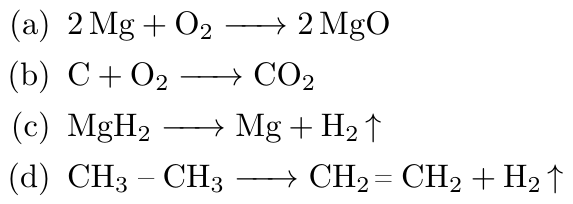
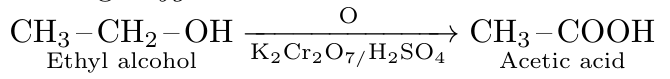

fvvv
LikeLike